Limestone is a sedimentary rock that has been used since ancient times for construction, agriculture, and industry. It is composed mainly of calcium carbonate, but it also contains other minerals that give it unique properties. In this article, we will explore the main components of limestone, their properties, and how they contribute to the formation and use of this versatile rock.
Chemical Composition of Limestone
The primary component of limestone is calcium carbonate (CaCO3), which makes up at least 50% of its composition. Calcium carbonate is a mineral that forms from the shells of marine organisms such as coral and mollusks. These organisms extract calcium and carbon dioxide from seawater to form their shells, which eventually accumulate on the ocean floor and become buried over time.
In addition to calcium carbonate, limestone may also contain small amounts of other minerals such as silica, clay, and iron oxide. These minerals give limestone different colors and patterns, making it an attractive building material.
Properties of Limestone
Limestone has several unique properties that make it useful in construction, industry, and agriculture. Some of these properties include:
Durability: Limestone is a durable building material that can withstand weathering and erosion. Its dense structure makes it resistant to damage from water, wind, and chemicals, making it ideal for outdoor applications such as building facades, walls, and pavements.
Porosity: Limestone is a porous rock that allows water to seep through its surface. This property makes it useful for drainage systems and irrigation channels in agriculture.
Alkalinity: Limestone is an alkaline substance that can neutralize acidic soils. When crushed and spread over acidic soils, it can raise the pH level and make the soil more conducive to plant growth.
Formation of Limestone
Limestone forms through a process called sedimentation, which occurs when sediment, such as mud or sand, accumulates in layers at the bottom of bodies of water. Over time, the weight of the sediment compacts the layers and turns them into rock. If the sediment consists of calcium carbonate from marine organisms, it can form limestone.
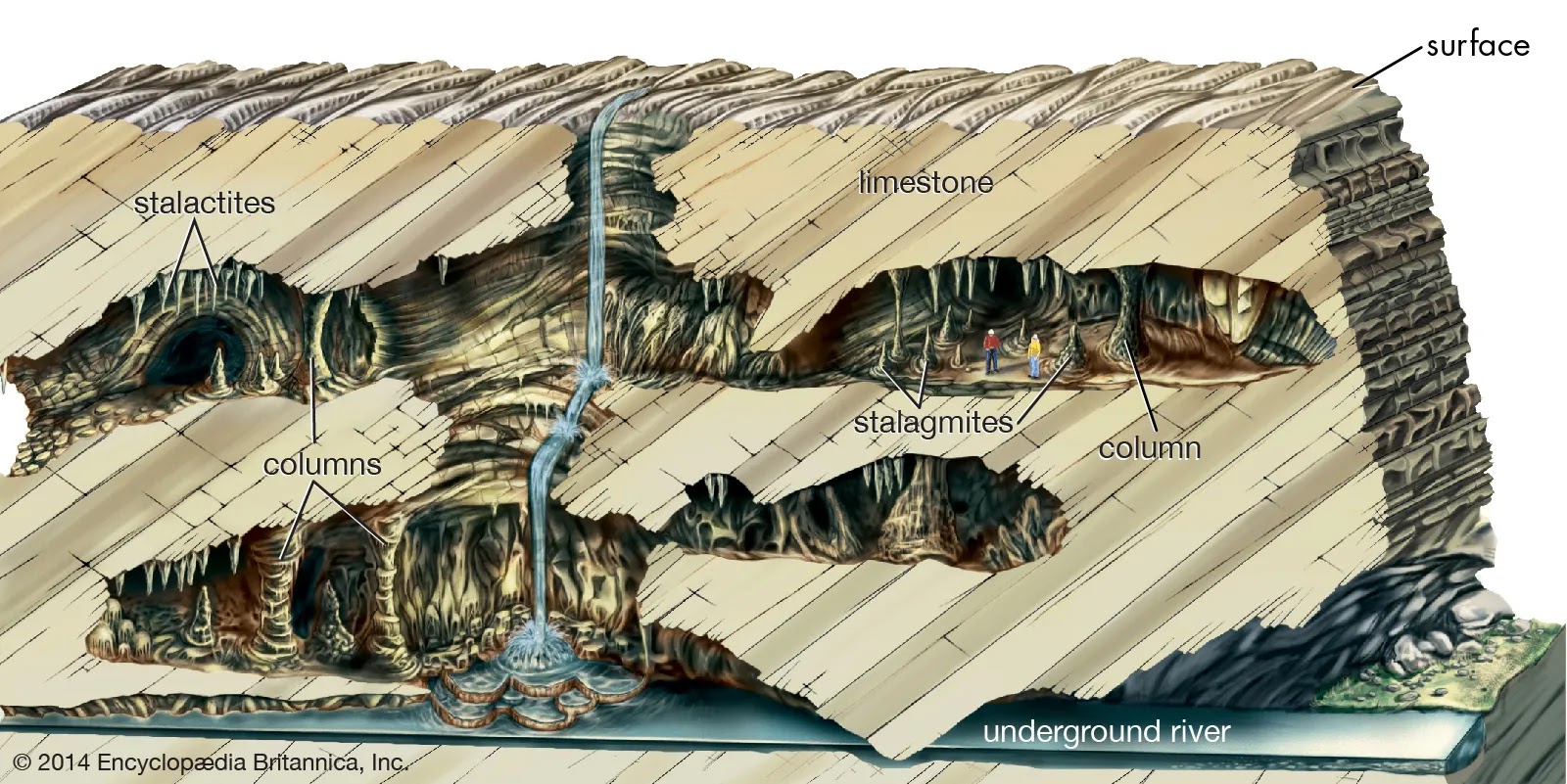
Limestone can also form through the precipitation of calcium carbonate from mineral-rich groundwater. This process occurs in caves and can result in the formation of stalactites and stalagmites.
Uses of Limestone
Limestone has several uses in various industries, including construction, agriculture, and manufacturing. Some examples include:
Construction: Limestone is a popular building material because of its durability, versatility, and aesthetic properties. It is used for building facades, walls, floors, and decorative elements such as columns and statues.
Agriculture: Limestone is used in agriculture to improve soil quality and increase crop yields. When added to acidic soils, it neutralizes the acidity and provides essential nutrients such as calcium and magnesium.
Manufacturing: Limestone is used in the manufacturing of cement, glass, and steel. In cement production, limestone is crushed and heated with other materials to produce clinker, which is then ground into a fine powder and mixed with gypsum to create cement. In glass production, limestone is used as a flux to reduce the melting point of silica, while in steel production, it is used as a flux to remove impurities and lower the melting point of iron.
Limestone is a versatile and valuable resource that has been used for thousands of years for construction, agriculture, and industry. Its primary component, calcium carbonate, gives it unique properties such as durability, porosity, and alkalinity, making it useful in a wide range of applications. Understanding the main components of limestone and their properties is essential for appreciating its contributions to our society and the environment. By studying limestone, we can gain important insights into the Earth's history and its processes.